PRODUCT CATEGORIES
CLASSES/REGISTRATION
WHAT'S YOUR ROLE?
It's FDA Renewal Time!
Are you confused about FDA registration? Don’t be! Applied takes the worry out of annual registration for you!
Transfillers need to annually renew their transfilling locations and drug listings with the FDA. Here are some quick tips to help you stay compliant.
Who needs to register?
Anyone who fills, or private labels, the gas must register. The FDA states that any firm that transfers oxygen from one container to another, via gas to gas, liquid to gas, or liquid to liquid; is considered a drug manufacturer by the FDA and is required to register with the FDA annually (See 21 CFR 207.21(a)).
When do I need to renew my transfill location’s FDA registration and drug listing?
The FDA requires drug manufacturers including transfillers to renew with the FDA every year between October 1st and December 31st of the year (See 21 CFR 207.21(a)).
When you are getting ready to register or renew with the FDA, remember these tips:
1. Your transfilling site address must match the address on file with Dun and Bradstreet, so be sure to check your DUNS number.
2. If you are filling different cylinder sizes since the last time you registered, your drug listing should be updated.
3. If you are no longer filling at a specific location, it is recommended that you remove the location from the FDA database.
4. Starting June 1, 2009, the FDA stopped accepting paper submissions for registration. Registration must now be done electronically.
To view your current FDA registration on the FDA’s website, click here.
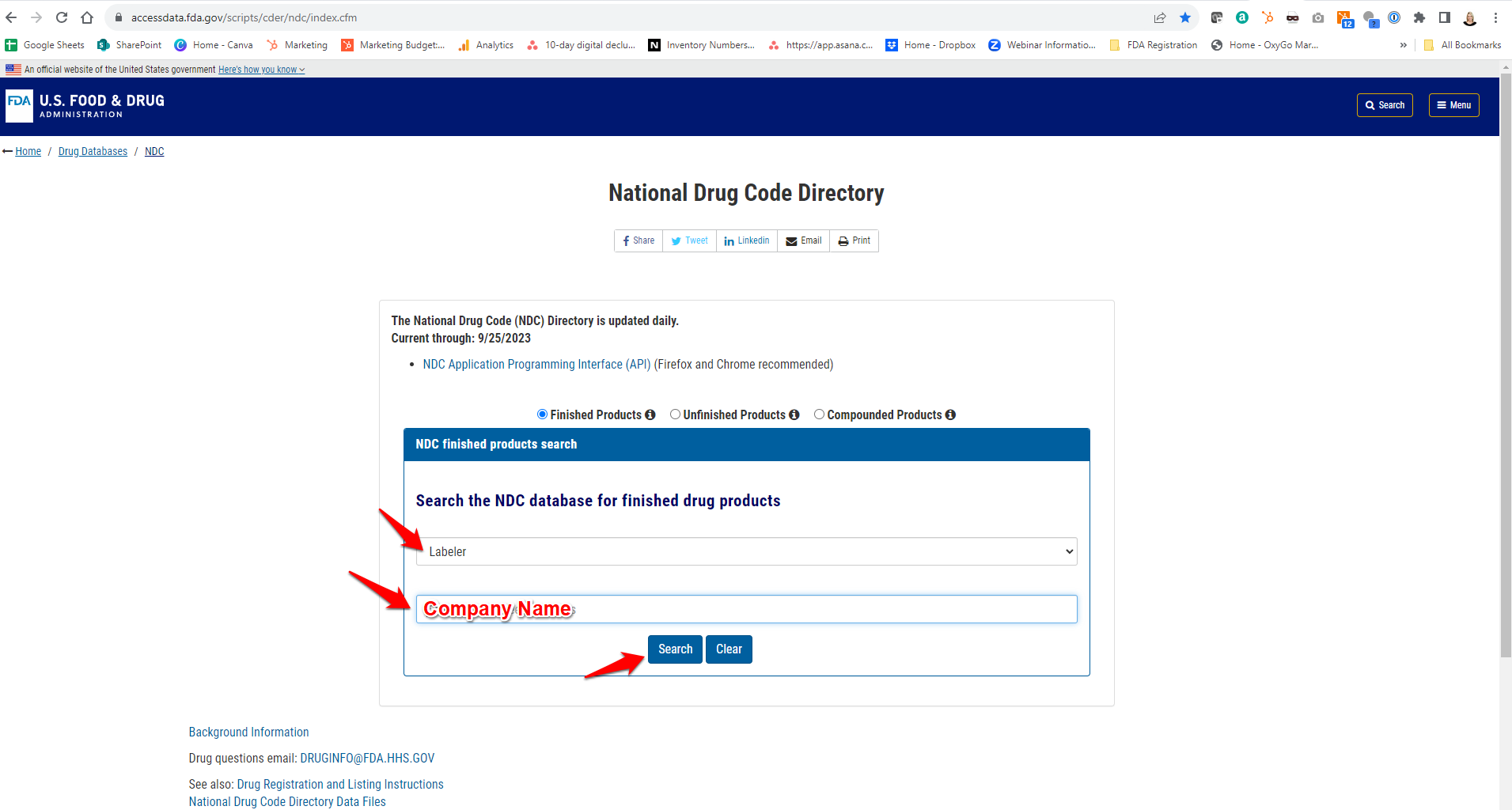
To view your current FDA drug listing, click here and select “Labeler” in the drop-down menu before typing your company name and searching.
To complete our renewal form, visit www.applied-inc.com/fda.
For questions, contact us at registration@applied-inc.com.
You Might Also Like
Subscribe to our Newsletter
Get the latest regulatory info, accreditation news and exclusive discounts!
 View Cart []
View Cart []