PRODUCT CATEGORIES
CLASSES/REGISTRATION
WHAT'S YOUR ROLE?
Latest News
Month: September 2013
Who needs training?
In any manufacturing operation involving human manipulation there are hazards which may be created by preoccupation, mental lapse, carelessness and the like. Therefore, all on-the-job and CGMP training should be revisited at frequent intervals and needs to be conducted by qualified individuals. A firm is expected to establish detailed written procedures (training program) outlining the specific areas of the firm’s operation to be covered. On-the-job training is acceptable, as long as the training is conducted by a qualified individual on a frequent basis. Any employee involved in the manufacturing, filling, processing, handling, holding, or shipping of a medical drug is required to be trained both on-the-job and in the current good manufacturing practices. This would include all delivery and/or truck drivers who deliver medical drug products. The lack of CGMP training is one of the most overlooked areas observed at most medical gas firms. CGMP training should be conducted by qualified individuals on a continuing basis and with sufficient frequency to assure that employees remain familiar with CGMP requirements that are applicable to their function. Conducting CGMP training once a year is not recommended, but instead should be presented in smaller more manageable portions, presented throughout the year with documentation* of the type, time, and attendance of each session. Applied offers CGMP training books and online classes to help get
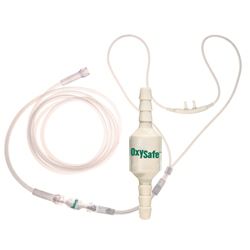
How to Stop Patient Cannula Fires
How OxySafe Firebreak stops a patient oxygen cannula fire. OxySafe is the same device as a
Training Time!
When oxygen providers need training for their employees, what training and how often.
Its in the Bag! Patient Cylinder Bags
Review of Patient oxygen cylinder bags for M6 cylinders, D and and Helios liquid portable unit
New GHS Oxygen Label and Training Requirements from OSHA
Get your On Demand New GHS Label Training from
What Cleaner Should I Use For My DME?
What Cleaner to Use for Oxygen Durable Medical Equipment and Medical Oxygen Cylinders
What is a QCU?
The FDA requires each firm to establish a quality control unit (QCU). The QCU has the responsibility and authority to approve or reject all drug product containers, closures, in-process materials, labeling, written procedures, the authority to review production records to assure completeness and accuracy, and is responsible for the approval or rejection of all manufactured drug products. The responsibilities and procedures applicable to the QCU must be in writing and must be followed. The designated person(s) are required to meet the Personnel Qualification requirements [211.25], must be identified in the firm’s procedures, and must receive appropriate quality assurance training. This includes training in the particular operations that the employee performs and in current good manufacturing practice as it relates to the employee's functions. A small firm may designate a single individual with the personnel qualifications. QCU managers and personnel can get the training they need fast and easy with Applied's QCU 101 Online
Getting more from your oxygen business
Tips, tricks and details on filling your own oxygen, and doing better in the oxygen transfilling business
What is cGMP and why is it important?
Learn what cCMP means and why it is important when you are an Oxygen
Subscribe to our Newsletter
Get the latest regulatory info, accreditation news and exclusive discounts!
 View Cart []
View Cart []