Posted By Kate Wildenthaler
January 16, 2025
Category: , , , General
Ensure compliance with DOT regulations for home medical oxygen transport. Learn the key requirements for vehicles, documentation, and placarding to stay safe and avoid
Posted By Kate Wildenthaler
October 25, 2024
Category: General
Ensure safety and reliability of oxygen equipment with proper cleaning and leak testing. Learn essential steps to prevent
Posted By Kate Wildenthaler
October 16, 2024
Category: General
Ensure safety and DOT compliance in oxygen transport with tips on log books, cylinder securing, and placarding. Stay informed to protect your business and
Posted By Laura Frederick
August 03, 2021
Category: General
Applied and OxyGo are now members of Big Sky
Posted By Laura Frederick
October 28, 2020
Category: General
Drivers, respiratory therapists, warehouse personnel, and others who handle compressed gas cylinders should do so using the proper equipment to ensure safe movement of the
Posted By Laura Frederick
September 09, 2020
Category: General
Start getting more from you oxygen business with Applied's liquid to gas and gas to gas
Posted By Brittany Fichter
May 20, 2020
Category: General
Cut your oxygen costs by up to 90% with Applied's refilling systems. We guarantee at least 25% savings per cylinder. Contact us to learn
Posted By Laura Frederick
April 07, 2020
Category: General
Ensure safety when filling medical oxygen cylinders by following critical pre-fill checks, including odor testing and visual
Posted By Brittany Fichter
December 13, 2019
Category: General
Join OxyGo's Oxygen EXPO2 on January 21st, 2020 in Orlando! Learn from industry experts on the future of ambulatory oxygen & tech
Posted By Brittany Fichter
November 19, 2019
Category: General
Travel effortlessly with the FAA-compliant OxyGo NEXT, featuring extended battery life, Bluetooth connectivity, and
Posted By Victoria Marquard-Schultz
June 11, 2014
Category: General
the different between 1/2\\\\\" and 1/4\\\\\" NPT for medical oxygen industry
Posted By Kristen Cifranic
April 11, 2014
Category: General
OF-700 service plan, Andonian, HP-40, transfilling tech help
Posted By Victoria Marquard-Schultz
February 27, 2014
Category: General
Applied has created a full day seminar covering how to handle, transport and fill oxygen- as well as other accreditation topics to help home care providers keep up with changing regulatory and technical
Posted By Victoria Marquard-Schultz
January 15, 2014
Category: General
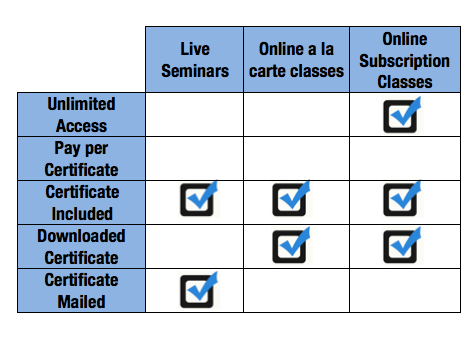
Applied Training Frequently Asked Questions for online classes, training workbooks and live seminars.
Posted By Victoria Marquard-Schultz
January 13, 2014
Category: General
Frequently asked questions about Applied\'s Live Regional Seminars, including why they are needed, who should take them, what they cover and
Posted By Victoria Marquard-Schultz
November 27, 2013
Category: General
Posted By Kristen Cifranic
November 20, 2013
Category: General
Maintaining your oxygen equipment: 3 easy steps includes gauges and thermometers, Servomex, pigtails and
Posted By Kristen Cifranic
November 06, 2013
Category: General
According to the CGMPs, each manifold filling sequence, each uninterrupted filling sequence, every cryogenic vessel filled, and each storage tank following a delivery is considered a new lot and is required to be assigned a new lot number.
For firms filling liquid oxygen for delivery to home patients, each of the large cryogenic vessels or dewars either portable or permanently mounted in a van or a truck are required to be assigned a unique lot number.
The assigning of a single lot number for an entire day's production is not acceptable. A manufacturing operation, such as the filling of high pressure cylinders on a multi-outlet manifold, is governed by a set of manufacturing procedures or conditions. When these procedures are performed from the beginning to the end of a process they provide assurance that the batch is uniform and consistent. As such, each batch is in itself a separate entity with its filling operations unique to that filling sequence.
At the present time, cryogenic home vessels filled at a patient’s home, i.e., curbside are not required to bear a lot number. However, cryogenic home vessels filled on site and stored for future delivery, or cryogenic home vessels filled by a third party, require lot numbers.
Click here to see Applied's lot
Posted By Kristen Cifranic
September 25, 2013
Category: General
In any manufacturing operation involving human manipulation there are hazards which may be created by preoccupation, mental lapse, carelessness and the like. Therefore, all on-the-job and CGMP training should be revisited at frequent intervals and needs to be conducted by qualified individuals.
A firm is expected to establish detailed written procedures (training program) outlining the specific areas of the firm’s operation to be covered. On-the-job training is acceptable, as long as the training is conducted by a qualified individual on a frequent basis.
Any employee involved in the manufacturing, filling, processing, handling, holding, or shipping of a medical drug is required to be trained both on-the-job and in the current good manufacturing practices. This would include all delivery and/or truck drivers who deliver medical drug products.
The lack of CGMP training is one of the most overlooked areas observed at most medical gas firms. CGMP training should be conducted by qualified individuals on a continuing basis and with sufficient frequency to assure that employees remain familiar with CGMP requirements that are applicable to their function. Conducting CGMP training once a year is not recommended, but instead should be presented in smaller more manageable portions, presented throughout the year with documentation* of the type, time, and attendance of each session.
Applied offers CGMP training books and online classes to help get
Posted By Victoria Marquard-Schultz
September 18, 2013
Category: General
What Cleaner to Use for Oxygen Durable Medical Equipment and Medical Oxygen Cylinders
 View Cart []
View Cart []