PRODUCT CATEGORIES
CLASSES/REGISTRATION
WHAT'S YOUR ROLE?
Latest News
Category: Regulatory Compliance
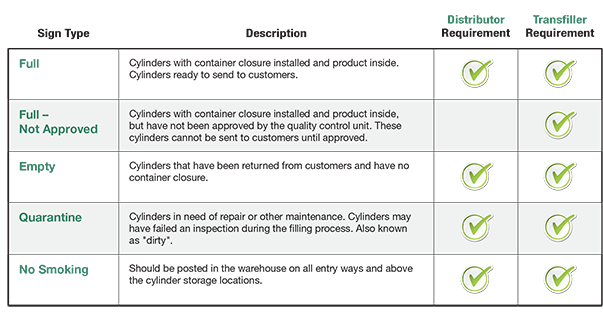
A Quick Guide To Warehouse Signage
Ensure proper storage and signage for medical oxygen, classified as a prescription drug by FDA and hazardous by DOT, to maintain
GHS Pictograms for Home Care Providers
OSHA, FDA and DOT have guidelines developed for precautionary labels for use on oxygen cylinders and cryogenic vessels. The appropriate pictogram must now be used on
Handy Calibration Chart From Applied
FDA required equipment calibration and maintenance schedule for oxygen transfillers of liquid to gas and gas to gas transfilling.
What does your accreditation company require at your site?
We have done a survey of the most popular accreditation companies, and here is what will most likely be required by your
Mini Oxygen Storage Audit
Take our mini storage audit to find out if your cylinders are being properly
NEW OSHA Requirements For Oxygen Cylinder Labels
Explanation of new OSHA requirements for medical oxygen drug product
New Labeling and Hazcom Training Requirements
GHS, or Globally Harmonized System of Classification of Labeling of Chemicals, is a system used world wide to standardize the labeling and safety data sheets for chemicals. Because there are so many chemicals and hazardous substances used world wide, GHS strives to make everyone safer by creating a universal communication system. THe GHS is not a regulation body in and of itself, but rather a set of standards that regulatory bodies can adopt to create a uniform system across the globe. Think of GHS as akin to the metric or imperial system- metric or imperial are not themselves a regulation, but something that regulating bodies adopted. Similarly, GHS has recently been adopted by OSHA, the Occupational Safety and Health Administration of the Federal Government, to standardize hazardous material safety in the US. This means that the way things look on the chemicals you use and the material safety data sheets you see will change. Each company will need to train employees on the key elements of the GHS system to meet OSHA standards. Here's a breakdown of how this applies to Oxygen Providers: 1. You'll have to train your employees how to read the new SDS (Safety Data Sheet) format by December 1, 2013. The new SDS format requires 16 standardized
Labels
FDA Drug Product Labels: PACKAGING and LABELING CONTROL Sections 211.122, 125, & 130 A firm must establish written labeling procedures covering the receipt, identification, storage, handling and examination of all labeling. Procedures should cover the reconciliation, issuance, returns, and security of labeling. There must be written procedures to assure that the correct label is used on the drug product, including the identification of each batch with a lot number. All high pressure cylinders, large cryogenic vessels and cryogenic home vessels are required to bear an adequate drug product label. This label is usually the responsibility of the manufacturer, filler, transfiller, etc. There can be only one drug label on a high pressure cylinder or cryogenic vessel, and that label is usually applied by the manufacturer, filler, transfiller, etc. of the drug product. Cryogenic home vessels come from the manufacturer with a DEVICE label, which should not be confused with the required drug label. Further, the device label must not be removed. Cylinders owned by one company, but filled by another company may bear a small ownership or possession sticker in addition to the drug product label. Upon receipt from the printer, a new batch of labels must be counted to verify the quantity of labels received, and they should be examined and compared against the approved master label to assure correctness. If a firm uses a small, grocery-store type
What is a QCU?
The FDA requires each firm to establish a quality control unit (QCU). The QCU has the responsibility and authority to approve or reject all drug product containers, closures, in-process materials, labeling, written procedures, the authority to review production records to assure completeness and accuracy, and is responsible for the approval or rejection of all manufactured drug products. The responsibilities and procedures applicable to the QCU must be in writing and must be followed. The designated person(s) are required to meet the Personnel Qualification requirements [211.25], must be identified in the firm’s procedures, and must receive appropriate quality assurance training. This includes training in the particular operations that the employee performs and in current good manufacturing practice as it relates to the employee's functions. A small firm may designate a single individual with the personnel qualifications. QCU managers and personnel can get the training they need fast and easy with Applied's QCU 101 Online
Subscribe to our Newsletter
Get the latest regulatory info, accreditation news and exclusive discounts!
 View Cart []
View Cart []